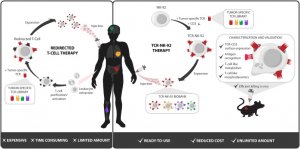
Cancer Crosslinks 2020
 Oslo Cancer Cluster
Oslo Cancer Cluster
Engaging presentations by leading international and Norwegian oncology experts at the 12th Cancer Crosslinks “Progress in Cancer Care – A tsunami of promises or Game Changing Strategies?”.
Oslo Cancer Cluster’s annual meeting gathered more than 350 delegates from all over Norway at the Oslo Cancer Cluster Innovation Park, and more than 50 participants followed the live stream. The record high participation shows the large interest in translational cancer research and the importance of the programme for the Norwegian oncology community.
Cancer Crosslinks has become one of the largest national meeting places for oncologists, haematologists, translational researchers, regulatory experts and industry representatives. The meeting offers a full day educational program.
The aim of the conference is to stimulate broader interactions between researchers and clinicians, to encourage translational and clinical research, and to inspire collaborations. Novel partnerships between industry, academia and authorities are essential to deliver new treatments and diagnostics to Norwegian cancer patients.
“At the start of 2020, cancer patients have more treatment options than ever before. Immuno-oncology is firmly established as the fourth pillar of cancer treatment and the tremendous progress in the field is reflected in increased survival rates,” said Jutta Heix, Head of International Affairs, Oslo Cancer Cluster. “However, many patients do not benefit from novel treatments and we still have significant gaps in our understanding of the complex biological mechanisms. Deciphering this complexity is a task for the decade to come. The Cancer Crosslinks 2020 speakers are shedding light on emerging concepts and key challenges and discuss how they are addressing them to advance cancer care.”
An inspiring programme
Referring to a record number of new oncology drug approvals in recent years and an enormous global pipeline of drugs in late-stage development, this year’s programme addressed the question “Progress in Cancer Care – A Tsunami of Promises or Game-Changing Strategies?”. Distinguished international experts from leading centres in the US and Europe presented emerging concepts, recent progress and key questions to be addressed for both solid and haematological cancers.
Cancer researchers and clinicians from all of Norway enjoyed excellent presentations and engaging discussions with speakers and colleagues.
“Cancer Crosslinks 2020 gave me an opportunity to listen to talks by international top scientists, and discuss some of the latest developments in translational cancer research with meeting participants from academia and industry in a relaxed and inspiring setting,” said Johanna Olweus, Head of Department of Cancer Immunology at the Institute for Cancer Research.
“Cancer Crosslinks is always a meeting that makes me proud of being part of Oslo Cancer Cluster. It is inspiring to see Norwegian and international participants come together to discuss recent progress in cancer research and how to develop cancer treatments for the patients,” said Øyvind Kongstun Arnesen, Chairman of the Board, Oslo Cancer Cluster.
The day programme was complemented with an evening reception in the city center where speakers and delegates continued their lively discussions and listened to an inspiring talk by Ole Petter Ottersen, President of Karolinska Institute, at Hotel Continental in Oslo.
Cancer Crosslinks was established by Oslo Cancer Cluster in 2009 in collaboration with the pharmaceutical company Bristol-Myers Squibb.
“Cancer Crosslinks 2020 has been a fantastic conference, where the presenters have given an excellent description of current and near future achievements within cancer research and the importance of understanding the underlying biology of cancer to rationally give patients the correct cancer therapy. In particular within immunotherapy, there is a need to understand the dynamic complexity of tumor immunology and how to apply the best and tailored immuno-oncology based treatment strategy for cancer patients,” said Ali Areffard, Disease Area Specialist Immuno-Oncology, Bristol-Myers Squibb.
This year, the pharmaceutical company Sanofi Genzyme Norway was a proud co-sponsor of the meeting.
“It was great to be able to provide a platform for interaction between the Norwegian scientific cancer environment and top international research capacities. Therefore, it was with huge enthusiasm Sanofi Genzyme co-sponsored this important conference. New treatment options in oncology are developing fast, where new treatment modalities provide clinicians with additional and superior options. New treatments specifically targeting the malignant cells, as well as activating the host immune response towards the cancer, provides tools to significantly improve current cancer treatments. This year’s Cancer Crosslinks conference gave an excellent insight into this,” said Knut Steffensen, Medical Advisor Hematology Nordic & Baltics, Sanofi Genzyme.
Interview with Prof. Jason Luke
View the interview with Prof. Jason Luke, by HealthTalk, in the video below:
Interview with Prof. Michel Sadelain
View the interview with Prof. Michel Sadelain, by HealthTalk, in the video below:
The speakers at Cancer Crosslinks 2020

Jason J. Luke, MD, FACP, Director of the Cancer Immunotherapeutics Center, Associate Professor of Medicine, University of Pittsburgh Medical Center and Hillman Cancer Center, USA. Photo: Cameo UB Productions/Oslo Cancer Cluster

Stefani Spranger, Howard S. and Linda B. Stern Career Development Assistant Professor, Koch Institute for Integrative Cancer Research at MIT, Cambridge, USA. Photo: Cameo UB Productions/Oslo Cancer Cluster

Harriet Wikman, Professor, Group Leader, Center for Experimental Medicine, Institute of Tumor Biology, University Medical Centre Hamburg-Eppendorf, Germany. Photo: Cameo UB Productions/Oslo Cancer Cluster

Vessela Kristensen, Head of Research and Development and Director of Research at the Dept. of Medical Genetics, Oslo University Hospital, Norway. Photo: Cameo UB Productions/Oslo Cancer Cluster

Peter A. Fasching, Professor of Translational Gynecology and Obstetrics, University Hospital and Comprehensive Cancer Center Erlangen-EMN, Germany. Photo: Cameo UB Productions/Oslo Cancer Cluster

Karl Johan Malmberg, Professor, Group Leader Dept. of Cancer Immunology and Director STRAT-CELL, Oslo University Hospital, Norway. Photo: Cameo UB Productions/Oslo Cancer Cluster

Michel Sadelain, MD, PhD, Professor, Director, Center for Cell Engineering, Memorial Sloan Kettering Cancer Center, New York, USA. Photo: Cameo UB Productions/Oslo Cancer Cluster

Bjørn Tore Gjertsen, Professor of Hematology, Centre for Cancer Biomarkers CCBIO, Dept. of Clinical Science, University of Bergen, Norway. Photo: Cameo UB Productions/Oslo Cancer Cluster

Hermann Einsele, Professor, Chair, Dept. of Internal Medicine II, Head of the Clinical and Translational Research Program on Multiple Myeloma, University Hospital Wuerzburg, Germany. Photo: Cameo UB Productions/Oslo Cancer Cluster