Natural killer cells dressed to kill cancer cells

New research: A new study may potentially enable scientists to provide cancer immunotherapy that is cheaper, faster and more manageable.
New work by researchers with laboratories at Oslo Cancer Cluster Incubator may help to dramatically improve a T cell-based immunotherapy approach so that it can benefit many more patients.
T cell assassins
T cells are the professional killers of the immune system – they have a unique capability to specifically recognize ‘foreign’ material, such as infected cells or cancer cells. This highly specific recognition is achieved through receptors on the surface of T cells, named T cell receptors (TCRs). Once its receptor recognizes foreign material, a T cell becomes activated and triggers the killing of the infected or cancerous cell.
Adoptive cell therapy
Unfortunately, many cancers have adapted fiendish ways to avoid recognition and killing by T cells. To combat this issue, an immunotherapy approach known as adoptive cell therapy (ACT) has been developed in recent years. One such ACT approach is based on the injection of modified (or ‘re-directed’) T cells into patients. The approach is further explained in the illustration below.
The left side of the illustration shows how redirected T-cell therapy involves:
1) Harvesting T cells from a cancer patient
2) Genetic manipulation of T cells to make them express an ideal receptor for recognizing the patient’s cancer cells
3) Growing T cells in culture to produce high cell numbers
4) Treating patients with large quantities of redirected T cells, which will now recognize and kill cancer cells more effectively
An alternative approach
Adoptive T cell therapy has delivered very encouraging results for some cancer patients, but its application on a larger scale has been limited by the time consuming and costly nature of this approach. In addition, the quality of T cells isolated from patients who have already been through multiple rounds of therapy can sometimes be poor.
Researchers have long searched for a more automated form of adoptive cell therapy that would facilitate faster and more cost-effective T cell-based cancer immunotherapy.
One approach that has seen some success involves the use of different immune cells called Natural Killer cells – NK cells in brief.
Despite their great potential, NK cells have unfortunately not yet been proven to provide a successful alternative to standard T cell-based cancer immunotherapy. One major reason for this may be that, because NK cells do not possess T cell receptors, they are not very effective at specifically detecting and killing cancer cells.
Cells dressed to kill
The group led by Dr. Sébastien Wälchli and Dr. Else Marit Inderberg at the Department of Cellular Therapy aimed to address this issue and improve NK cell-based therapies.
They reasoned that by editing NK cells to display anti-cancer TCRs on their cell surface they could combine the practical benefits of NK cells with the potent cancer killing capabilities of T cells. This is shown in the right hand side of the illustration above.
The researchers found that by simply switching on the production of a protein complex called CD3, which associates with the TCR and is required for T cell activation, they could indeed induce NK cells to display active TCRs. These ‘TCR-NK cells’ acted just like normal T cells, including their ability to form functional connections to cancer cells and subsequently mount an appropriate T cell-like response to kill cancer cells.
This was a surprising and important finding, as it was not previously known that NK cells could accommodate TCR signaling.
This video shows TCR-NK cell-mediated killing of cancer cells in culture. The tumour cells are marked in green. Tumour cells that start dying become blue. The overlapping colours show dead tumour cells.
The researchers went on to show that TCR-NK cells not only targeted isolated cancer cells, but also whole tumours.
The method was proven to be effective in preclinical studies of human colorectal cancer cells in the lab and in an animal model. This demonstrates its potential as an effective new form of cancer immunotherapy.
Paving the way
Lead researcher Dr. Nadia Mensali said:
“These findings pave the way to the development of a less expensive, ready-to-use universal TCR-based cell therapy. By producing an expansive ‘biobank’ of TCR-NK cells that detect common mutations found in human cancers, doctors could select suitable TCR-NK cells for each patient and apply them rapidly to treatment regimens”.
Whilst further studies are needed to confirm the suitability of TCR-NK cells for widespread treatment of cancer patients, the researchers hope that these findings will be the first step on the road towards off-the-shelf immunotherapy drugs.
- Read the whole research paper at Science Direct. The paper is called “NK cells specifically TCR-dressed to kill cancer cells”.
- The researchers behind the publication consists of Nadia Mensali, Pierre Dillard, Michael Hebeisen, Susanne Lorenz, Theodossis Theodossiou, Marit Renée Myhre, Anne Fåne, Gustav Gaudernack, Gunnar Kvalheim, June Helen Myklebust, Else Marit Inderberg, Sébastien Wälchli.
- Read more about research from this research group in this article from January.
- Read more about Natural Killer cells in this Wikipedia article.
The Incubator Labs are expanding

The laboratories at Oslo Cancer Cluster Incubator are expanding to meet increasing demand from members.
Oslo Cancer Cluster Incubator has recently converted three offices into new laboratories to accommodate the rising demand from their members.
From the opening in 2015, the laboratories in the Incubator have been a great success. Several of the start-ups have expanded their work force and require more offices and lab space.
The new laboratory is jointly occupied by Zelluna Immunotherapy and the Department of Cellular Therapy (Oslo University Hospital). The Institute for Energy Technology and Arctic Pharma have also expanded their laboratories with an extra room each.
The laboratories are now running at full capacity, but there is some space available in the shared labs. Some of the members of the Incubator offer their services to outside companies who are in need of getting lab work done.
“Our ambition is to grow the Incubator Labs further into the new Innovation Park next door.” Bjørn Klem, General Manager
The Incubator occupies over 550 square meters. Offices have been converted into labs to meet the growing interest from the members.
A unique model
The Incubator Labs follow a unique model, which offers both private laboratories and fully equipped shared laboratories. The private laboratories are leased with furniture, water supply, electricity and ventilation. The companies bring their own equipment depending on their needs.
Shared laboratories, including a bacteria lab, a cell lab and wet lab, are leased including basic equipment with the opportunity for companies to bring their own if shared by all tenants. All laboratories share the common support facilities including a cold room for storage, a laundry room, and storage room including cell tanks and nitrogen gas.
“This model of a shared laboratory is very unusual,” said Janne Nestvold, Laboratory Manager at the Oslo Cancer Cluster Incubator.
The advantage of working in a shared lab is that companies can avoid the costs and limitations associated with setting up and managing a laboratory. A broad range of general equipment, including more advanced, analytical instruments, are provided by the Incubator.
”It would be too expensive for a small company to buy all this equipment themselves.” Janne Nestvold, Laboratory Manager

The Department of Cellular Therapy (Oslo University Hospital) are one of the members using the shared lab. Photograph by Christopher Olssøn
Open atmosphere
The laboratories have an open and light atmosphere. Large windows provide ample lighting and all spaces are kept clean and tidy. The halls are neatly lined with closets and plastic containers for extra storage.
The general mood is calm and friendly. Nestvold communicates daily with the users about changes, updates and improvements, which sets an informal tone. Thanks to monthly lab meetings, the users are also involved in the decision-making process. The companies often work side-by-side or in teams, fostering collaboration rather than competition. There is therefore a strong workplace culture based upon flexibility and mutual respect.
The companies often work side-by-side or in teams, fostering collaboration rather than competition.
Nestvold also ensures that the high demands on the infrastructure of the laboratory are met. She has put agreements in place to facilitate the members’ needs, such as the washing of lab coats, pipette service and shipping packages on dry ice. With all these services included, the Incubator Labs are attractive for researchers and companies to carry out their cancer research.
Over the years, Nordic Nanovector, OncoInvent, Targovax, Intersint, OncoImmunity have conducted research in the laboratories. Now, Arctic Pharma, the Department of Cellular Therapy (Oslo University Hospital), GE Healthcare, the Institute for Energy Technology, Lytix BioPharma, NorGenotech, Ultimovacs and Zelluna Immunotherapy are using the Incubator Labs to develop their cancer treatments.
- For more information about the Incubator Lab, get in touch with Janne Nestvold.
Attracting clinical trials to Norway

Dr. Jon Amund Kyte at Oslo University Hospital (OUH) and Oslo Cancer Cluster share the common goal of bringing more clinical trials to Norway.
Jon Amund Kyte is the new Head at the Department of Experimental Cancer Treatment at OUH. He also runs three separate clinical trials and is the leader of a research group at the Department of Cancer Immunology, where he develops novel CAR T cell therapy and conducts translational studies.
Kyte aims to increase the number of and improve the quality of clinical trials in Norway. He says this will contribute to more patients gaining access to novel cancer treatments and to improving the efficacy of cancer therapies.
“The only way to improve cancer treatment is to have clinical trials,” said Kyte.
Oslo Cancer Cluster also wants to bring more clinical trials to Norway to develop innovative cancer medicines. The ambition is to enable faster patient recruitment from across the Nordic region, so that many more can benefit from new treatments, such as immunotherapy.
Promising advances
Immunotherapy represents a new type of cancer treatment, which activates the patient’s immune-system to fight off the cancer cells. It gives doctors the opportunity to help patients that previously had limited treatment options. Most types of immunotherapy also cause less side effects than traditional cancer treatments.
“The important point is that immunotherapy can have a long-term effect,” said Kyte.
“Most patients that experience a recurrence or progression of the disease cannot be cured. The traditional treatments only have a limited, short-term effect on them. But immunotherapy may have a long-term effect on the patient – and, in some cases, even cure the disease.”
Two big challenges
Immunotherapy may sound like a miracle drug, but researchers still have a long way to go to perfect the treatment for all cancer patients. Kyte highlights two of the biggest barriers that remain.
“One challenge is to develop immunotherapy so that it works efficiently on all types of cancer. The other challenge is to learn how to choose personalised treatment plans: to identify an individual’s biomarkers and find out which treatment will be effective for that specific patient.”
A biomarker is a biological molecule in the patient’s body and these may be used to see how well a patient will respond to a certain treatment. Kyte said that to develop immunotherapy, there needs to be more clinical trials. It is the only way for researchers to find out how to activate an immune response in the patient’s body.
“A big potential for development lies in trying different possible combinations of cancer treatments. In my clinical trials, for example, we combine immunotherapy with immunogenic chemotherapy or radiation therapy,” Kyte explained.

The Clinical Trial Unit are experts in assisting companies and researchers to conduct clinical trials in Norway.
Welcome, companies
OUH has a long history of conducting clinical trials and is an appealing option for both researchers, doctors and companies that wish to initiate their own trials. Kyte welcomes more companies to conduct clinical trials at OUH:
“The more clinical trials that are conducted here by companies, the stronger our clinical research environment becomes and our ability to run our own studies is also strengthened.”
The Clinical Trial Unit in Kyte’s department offers its services to companies that want to run a clinical trial at OUH. They have extensive background knowledge of how the hospital is organised and which approvals are needed to conduct a clinical trial in Norway. They can step in as project coordinator for companies that need help to get their clinical trials up and running.
“We are highly experienced in applying for approvals in Norway. When you run a clinical trial, there are regulations from the Norwegian Medicines Agency and the ethical committee and other governmental agencies. A clinical trial also involves many different parts of the hospital – the departments of pathology and radiology, the laboratories, the infusion unit, the hospital wards and out-patient clinic and the administrative offices that oversee different agreements, data management and biobanking.”
Nordic clinical trials
All these administrative obstacles may appear discouraging, but there are many convincing reasons to conduct a clinical trial in Norway.
“The Oslo University Hospital is a good place to run a clinical trial, because in terms of the number of cancer patients, it is one of the largest hospitals in Europe. Norwegian healthcare is also extremely well-organised. Patients are rarely lost to follow-up, because there are no private healthcare alternatives and patients rarely move out of the country,” Kyte explained.
The Clinical Trial Unit is also taking part in the development Nordic Nect, a collaboration to recruit patients from the entire Nordic region to clinical trials. The plan is to have one hospital where the clinical study is conducted and to involve patients from Sweden, Denmark, Finland and Norway. There will then be a population of 25 million people from which to recruit patients, which opens the possibility for larger clinical trials.
“This is a good thing for the companies that want to run clinical trials in Norway. It is also good for the researchers. But most of all, it is good for the patients – who have the opportunity to take part in more novel cancer treatments,” said Kyte.
- To learn more about the Clinical Trial Unit, please visit their website.
- Need help to get your clinical trial started? Get in touch with the Department of Experimental Cancer Treatment.

 OCC
OCC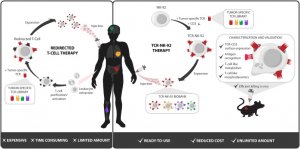

 OCC
OCC OCC
OCC

