Getting genomics into healthcare: look to the UK

During Cancer Crosslinks 2019, one thing was crystal clear: there is a need to include broader genomic testing into treatments for cancer patients in Norway.
“We are lacking behind here in Norway!”
Professor Ola Myklebost, from the Department of Clinical Science at the University of Bergen, was definitely ready for action in the panel debate at Cancer Crosslinks 2019, fittingly named “Call for Action”.
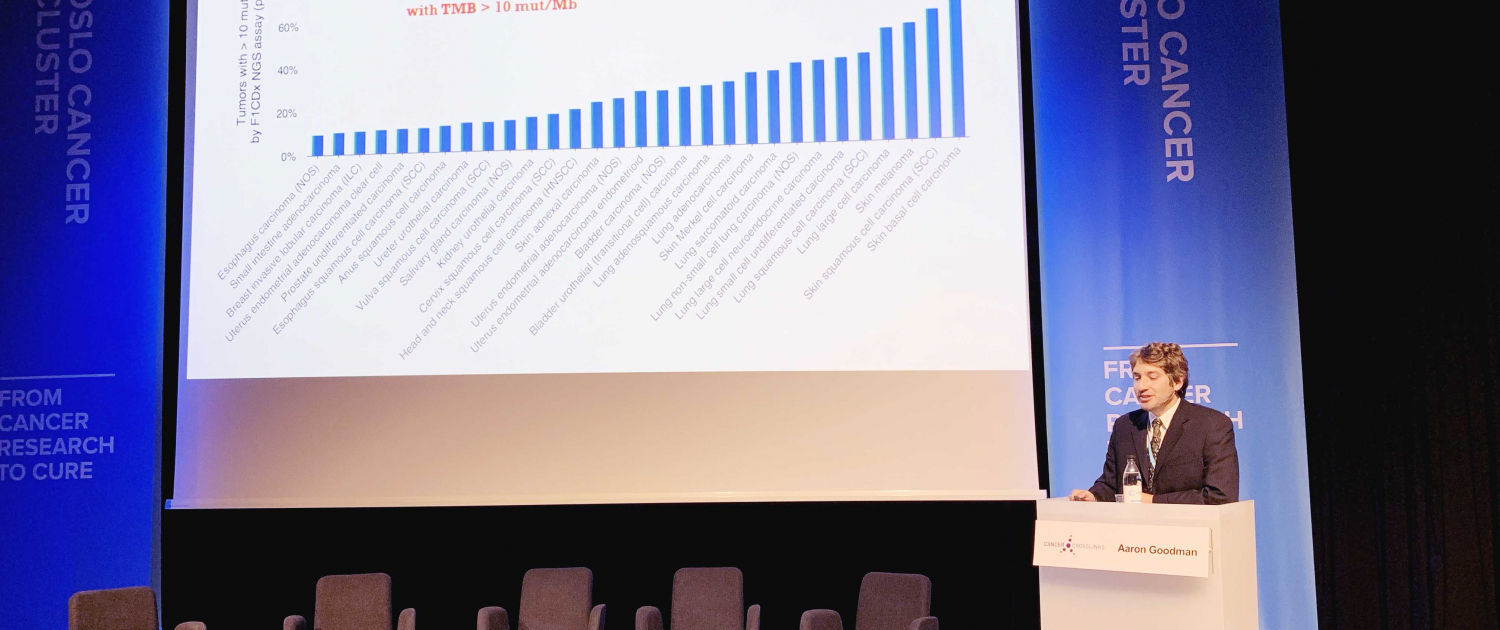
The panel and the audience of about 300 people had just listened to the talk given by James Peach. He is the Precision Medicine Lead at UK Medicines Discovery Catapult, Alderly Park, and prior to this, he was the Managing Director at the main programme for Genomics England from 2013 to 2017 and led the UK’s Stratified Medicines Program.
Peach told the audience how they have been implementing precision medicine into the public health care system (NHS) in the UK, using genomic testing, during the last decade. He demonstrated how the industry is part of this public endeavour, how political support and investment contributed to industry development, and how they addressed complex issues like sharing health data and using artificial intelligence.
It started with very little.
“In 2010, we had no structure”, Peach told the audience.

Sequencing 100,000 genomes
Thanks to all the British cancer patients who consented to Genomics England using their data, and a lot of common public-private efforts, Genomics England has now reached its goal of sequencing 100,000 whole genomes from NHS patients, according to their webpage. It takes a lot to accomplish this number, but luckily there are things to learn from the UK effort.
“Circulating tumour DNA testing is absolutely necessary”, Peach said from the podium.
The Life Science Sector deal from the British government outlines this public-private effort. It shows how significant government commitment, funding and strategic actions triggered investment and initiatives from the life science industry. You can read the entire document at the official webpage of the British Department of Business, Energy and Industrial Strategy, following this link.
James Peach visited Norway earlier as a speaker at Cancer Crosslinks 2012. Returning now, he was truly surprised about the current state of precision medicine in Norway.
Concerned about Norway
In an interview with Oslo Cancer Cluster, James Peach shared a concern as an answer to the question “What impressions are you left with after this conference?”
“It has left me quite concerned about the state of precision medicine in Norway. I thought you would be looking forward to the things you could do, but it turns out that there are actually some things that you should have done already.”
“Like what things?”
“Like universal application of a cancer panel test that is commercially feasible and deals around getting your data shared appropriately.”
“Do you think we can have a Genomics Norway?”
“Of course. It is probably about combining two things. One is that you got to get the basic stuff right. People need to have access to gene tests for their clinical care. Luckily the people here are a group of experts who are all connected to each other and who understand the system. It is not a massive system. I think there is a real chance to choose an area where Norway could do it exceptionally well. What that area is, is for you to choose.”
Concerns in Norway
Back in the panel discussion, Hege G. Russnes, Pathologist, Senior Consultant and Researcher at Oslo University Hospital, was getting involved:
“We need more information to help clinicians make therapy decisions. (…) Norway has no plan or recommendation for multi gene tests.”
Christian Kersten, Senior Consultant at the Center for Cancer Treatment at Sørlandet Hospital, agreed.
“I’m the clinician, I treat patients, patients die because of metastasis. I have been treating cancer patients for 20 years now and I feel it increasingly difficult to keep the trust of the patient.”
“If you ask the patients, they will sign the papers with consent of sharing data in 99% of the cases”, Myklebost added.
“We are only 5 million, we do not have to reinvent the wheel. Erna Solberg should invite James Peach for a cup of tea”, Christian Kersten said, finishing up the panel talk.
The entire panel debate is available to watch at the webcast webpage:
More on UK Medicines Discovery Catapult
Did this brief article make you interested in the work that James Peach and UK Medicines Discovery Catapult does? In this short video, Peach explains the challenges with access to health data for drug discovery and how to overcome them:
More from Cancer Crosslinks
We have more from Cancer Crosslinks 2019 coming up. Stay tuned and subscribe to our newsletter, and you will not miss videos of the talks and interviews with the other distinguished speakers at the conference.