Home testing to improve cancer prevention

Can home tests engage more people in cancer screening and save more lives?
“In Norway, 70% of all women are participating in the cervical cancer screening programme, which means 30% are not. Our goal is to include all and we need to get more women to participate,” said Sara Underland Mjelva, Leader of the Section for Prevention at the Norwegian Cancer Society.
This comment was made in a discussion about home testing during the webinar Results from the AnteNOR project: Norway’s way towards precision prevention at Oslo Cancer Cluster Innovation Park last week.
Reaching women at home
Home testing for HPV has been tested recently in Norway in a randomized clinical trial, led by Bo Terning Hansen, Senior Researcher at The Norwegian Institute of Public Health. The trial addressed women who hadn’t been screened for at least ten years.
“We found a huge difference in participation between the three control arms. Of those that just got the reminder letter, only 4% participated. While of those that received the test, 28% participated. That difference translates into a public health gain. We also found a huge difference in the diagnostic yield between the three arms,” explained Hansen.
This self-sampling device is now distributed by the Norwegian Cancer Registry and available in all GP offices in Norway. But what about other home tests?
Who pays for equal access?
Karl-Arne Johannessen, representing Tigeni, a company that offers home testing for cholesterol, long-term blood sugar, vitamins, and minerals, argued there are not enough financing solutions in Norway for home tests:
“If you go to the laboratory to take a test, the public healthcare pays for it, but there should also be public payment for the home tests in the future. It should be paid by the public if a doctor has asked you to take the test.”
Hansen agreed with Johannessen: “One thing is to get the tests funded, so everyone has equal access to them – that is the number one priority. There are also differences in ‘health literacy’ that you need to be aware of so you can compensate for that.”
“As a doctor myself, I support equal access to healthcare services. My experience is that currently public payers or healthcare organisations are quite conservative and cervical cancer is a good example. If we have a problem to engage individuals in preventive services, then home-based testing creates a more efficient additional option,” added Peeter Padrik, CEO of Antegenes, an Estonian healthcare company that develops PRS tests.
Predicting breast cancer
A PRS test is another kind of self-sampling device making headway in cancer screening. They are based on a technology called Polygenic Risk Score, which can provide a measure of someone’s personal risk to develop a disease. The Estonian-Norwegian collaboration project AnteNOR has for the last three years explored how to use these tests to improve prevention and early detection of breast cancer in Norway.

Peeter Padrik, CEO of Antegenes. Photo: Margit Selsjord/Oslo Cancer Cluster.
“The problem we are addressing is breast cancer, the most common cancer among women in Norway, Europe and worldwide. If we detect breast cancer as early as possible, then the treatment is very efficient,” said Padrik.
The breast cancer screening programme in Norway is for women aged 50-69, but 17% of breast cancer cases are among women younger than 50. It would not be reasonable to screen all women, so the challenge is to identify the younger women at risk.
Identifying genetic risk
“The genetic susceptibility is a very strong associated factor to those who develop breast cancer,” said Eivind Hovig, Professor at the Department of Bioinformatics at the University of Oslo. Hovig has published a study looking at how a combination of genetic markers indicate Norwegian women’s personal risk of developing breast cancer.

Eivind Hovig, Professor at the Department of Bioinformatics at the University of Oslo. Photo: Margit Selsjord/Oslo Cancer Cluster.
“Polygenic risk score is becoming a complementary instrument for risk stratification for various cancers. We find indeed that we can identify that there are individuals that have high risk to develop breast cancer,” Hovig added.
- Read more in the article New research on genetic risk and breast cancer.
The potential of PRS as a tool for risk-based mammography screening was also explored in a clinical pilot as part of the AnteNOR project. In total, 80 women aged 40-50 years took the PRS test to find out their personal risk of developing breast cancer.

Tone Hovda, senior radiologist at Vestre Viken. Photo: Margit Seljord/Oslo Cancer Cluster
“40 women were recommended to participate in BreastScreen Norway. 39 women were recommended to start mammography every second year before the age of 50. Among them, six were recommended to start annual mammography later on. One woman was recommended to start annual mammography from now on,” said Tone Hovda, senior radiologist at Vestre Viken and lead investigator for the clinical pilot.
- Read more in the article Results from breast cancer screening pilot.
Of European importance
Genetic tests are also a political priority on the EU level, as early detection and diagnosis are now possible for an increasing number of cancers with underlying heritable genetic risk.
There is now a call for “Accessible and affordable tests to advance early detection of heritable cancers in the European regions”. The budget includes 10-12 million euros per proposal and the submission deadline is 18 September. 2024
Sofia Anderholm Strand, Senior Adviser at the Research Council of Norway, presented the call: “They stress the need to validate easy-to-use, affordable and accessible genetic tests for early detection of cancer.”
A special thank you to all AnteNOR project partners for their contributions.

The AnteNOR project has been funded by:

The post Home testing to improve cancer prevention first appeared on Oslo Cancer Cluster.
Cell therapy breakthroughs in cancer treatment

The Section for Cellular Therapy’s Translational Research Unit in Norway has recently published two groundbreaking studies demonstrating the potential of cell-based therapies in the fight against cancer. The research group used the Oslo Cancer Cluster Incubator labs to develop pre-clinical treatments.
The collaborative team from Oslo University Hospital and the University of Bergen have made significant progress towards developing more effective and targeted treatments for advanced and treatment-resistant cancers.
Tackling colorectal cancer resistance
The first study, titled “Transient TCR-based T-cell therapy in a patient with advanced treatment-resistant MSI-high colorectal cancer,” focuses on a novel approach utilizing T-cell therapy to treat metastatic colorectal cancer. Published in a reputable journal, the study details the successful and safe application of a transient T-cell receptor (TCR) therapy named Radium-1 in a patient with advanced colorectal cancer resistant to conventional treatments. The therapy is named Radium-1, as it was discovered at the Radium Hospital (now part of Oslo University Hospital).
Dr Else Marit Inderberg, one of the lead researchers, explains the rationale behind their approach, stating,
“We aimed to modify the immune system to recognize and destroy tumour cells effectively. We transferred TCRs from responsive patients to non-responding ones, leading to promising results in our clinical trial.”
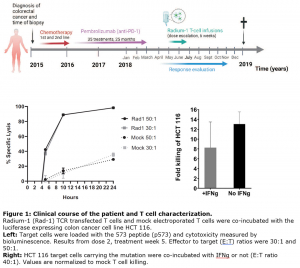
The therapy involves modifying a patient’s T cells to express the Radium-1 TCR using messenger RNA (mRNA) technology. Despite the challenging nature of advanced colorectal cancer, the treatment was well tolerated by the patient and resulted in stable disease, offering hope for further investigation in larger clinical trials.
Breakthroughs in ovarian cancer treatment
The second study, titled “Efficient CAR T cell targeting of the CA125 extracellular repeat domain of MUC16,” introduces a novel Chimeric Antigen Receptor (CAR) T-cell therapy targeting ovarian cancer. Ovarian cancer remains a significant challenge due to late-stage diagnosis and chemoresistance, making the development of new therapeutic strategies imperative.
Co-author Christopher Forcados and lead author Nicholas P. Casey. Photo: Oslo Cancer Cluster
Dr Nicholas P. Casey, the lead author of the study, emphasizes the importance of their research, stating, “Our CAR T-cell therapy targeting the CA125 extracellular repeat domain of MUC16 shows promising efficacy in preclinical models, offering a potential breakthrough in ovarian cancer treatment.”
The CAR T-cell therapy has shown promising results in both in vitro experiments and patient-derived xenograft mouse models. This paves the way for future clinical trials aimed at advancing CAR T-cell therapy for ovarian cancer patients. The development of these therapies took place in the fully-equipped laboratories of Oslo Cancer Cluster Incubator, specifically designed for carrying out these processes.
New hope and results ahead
These pioneering studies highlight the transformative potential of cell-based therapies in revolutionising cancer treatment. With further research and clinical validation, these innovative approaches could offer new hope for patients battling advanced and treatment-resistant cancers, and getting closer to realizing the vision of personalized and targeted cancer therapy.
As Dr Sebastien Wälchli, co-author of the studies, fittingly summarizes, “These findings underscore the importance of collaborative research efforts in advancing cancer immunotherapy and signify a significant step forward in our ongoing battle against cancer.”
The research group is anticipating the publication of a third article, which is expected to contain groundbreaking results. Stay tuned for further updates.
What is T-cell therapy for colorectal cancer:
Imagine your body has soldiers called T cells. These cells can recognize and fight against harmful things like cancer cells. In this therapy, scientists take T-cells from a patient’s body. Then modify these T-cells in a lab to make them better at recognizing and attacking cancer cells. It’s done by giving the T-cells a special weapon called Radium-1.
However, these modified T cells don’t stay in the patient’s body permanently. Instead, they are given back to the patient temporarily. These modified T cells go on a mission to find and destroy cancer cells. Even though they’re only there briefly, they can still make a big impact in fighting cancer cells.
What is CAR T-Cell Therapy for Ovarian Cancer:
In this therapy, scientists create special T cells called CAR-T cells. These T-cells are trained to recognize a specific target on ovarian cancer cells called MUC16. These CAR-T cells are specifically designed to find ovarian cancer cells.
In simpler terms, both therapies involve giving the body’s immune system a boost to help fight cancer more effectively. It’s like giving the immune system an upgraded set of tools to target and destroy cancer cells.
The post Cell therapy breakthroughs in cancer treatment first appeared on Oslo Cancer Cluster.
Lytix and Curida: Growth and innovation

Two companies from the Oslo Cancer Cluster have recently made remarkable achievements in both research and economic growth in the dynamic landscape of biotechnology and pharmaceuticals.
Lytix Biopharma and Curida, both Norwegian-owned, have gained attention and acclaim for their innovative approaches and substantial contributions to the healthcare sector.
Lytix Biopharma: Pioneering Immunotherapy
Lytix Biopharma, a clinical-stage immuno-oncology company based in Oslo, Norway, has been making waves with its research and development efforts in cancer immunotherapy. Spearheaded by CEO Øystein Rekdal, the company has recently secured between NOK 50 and 55 million in new capital, showcasing strong support from existing shareholders and new investors alike.
One of Lytix Biopharma’s flagship projects is the development of LTX-315, a novel cancer immunotherapy drug candidate currently undergoing Phase II studies. Partnering with Verrica Pharmaceuticals, Lytix Biopharma aims to revolutionize the treatment of skin cancer with LTX-315, offering patients a non-surgical option with potentially reduced risks and improved outcomes.
Rekdal highlights the significance of the partnership with Verrica, stating, “The collaboration with Verrica shows that the company’s drug candidate has a commercial potential in one of the largest cancer indications globally.” Moreover, Lytix Biopharma’s innovative approach, addresses key challenges in cancer therapy, promising enhanced efficacy and reduced side effects compared to current treatments.
Read more about Lytix drug candidate LTX-315
(In Norwegian)Les mer om Lytix Biopharma i Healthtalk
Curida: Empowering Pharma and Biotech
In parallel, Curida, another Oslo Cancer Cluster member company based in Oslo, Norway, has secured a significant growth investment from Signet Healthcare Partners and existing shareholders. This substantial investment, totalling between 230M NOK and 260M NOK, underscores Curida’s commitment to expansion and innovation in pharmaceutical manufacturing.
Ole J. Dahlberg, former CEO and Chairman of the Board at Curida, expressed enthusiasm about the partnership with Signet, stating, “We are excited by the opportunity to further accelerate growth and improve products for our pharma and biotech customers in their work to deliver medicines and therapeutics to benefit patients.”
The appointment of Anders Larsson as the new CEO further strengthens Curida’s leadership team, bringing extensive experience in management and strategic development from the pharmaceutical industry. With a focus on aseptic and non-aseptic liquid manufacturing, as well as biologics processing, Curida aims to cater to the evolving needs of pharmaceutical and biotech companies worldwide.
Read more in their press release
Plans for the Future
Both Lytix Biopharma and Curida have ambitious plans for the future, aiming to advance their respective projects and expand their impact on the healthcare industry. For Lytix Biopharma, upcoming studies at the Norwegian Radium Hospital hold promise for testing LTX-315 in earlier-stage melanoma patients, potentially broadening the reach of their innovative immunotherapy.
Rekdal shared their overarching goal, stating, “Our overall goal is to bring multiple projects forward and partner for late-stage development and commercialisation.” Similarly, Curida looks to leverage Signet’s industry expertise and investment to enhance its manufacturing capabilities and continue delivering high-quality solutions to pharmaceutical and biotech partners globally.
The post Lytix and Curida: Growth and innovation first appeared on Oslo Cancer Cluster.





